Abstract
Cellular therapies offer a promising therapeutic strategy for the highly malignant brain tumor, glioblastoma (GBM). However, their clinical translation is limited by the lack of effective target identification and stringent testing in pre-clinical models that replicate standard treatment in GBM patients. In this study, we show the detection of cell surface death receptor (DR) target on CD146-enriched circulating tumor cells (CTC) captured from the blood of mice bearing GBM and patients diagnosed with GBM. Next, we developed allogeneic “off-the-shelf” clinical-grade bifunctional mesenchymal stem cells (MSCBif) expressing DR-targeted ligand and a safety kill switch. We show that biodegradable hydrogel encapsulated MSCBif (EnMSCBif) has a profound therapeutic efficacy in mice bearing patient-derived invasive, primary and recurrent GBM tumors following surgical resection. Activation of the kill switch enhances the efficacy of MSCBif and results in their elimination post-tumor treatment which can be tracked by positron emission tomography (PET) imaging. This study establishes a foundation towards a clinical trial of EnMSCBif in primary and recurrent GBM patients.
Similar content being viewed by others
Introduction
After decades of advances in treatment strategies for different cancer types, highly malignant brain tumors, glioblastoma (GBM) still present a challenge for treatment1. Surgical resection followed by post-operative radio and chemotherapies are the current standard of care for GBM, but patients generally succumb to the disease1. Given the limited time from diagnosis to primary surgical intervention in GBM patients, stratification of therapy responders is becoming a critical aspect for providing patient-tailored biological therapies and ultimately improving GBM treatment. Circulating tumor cells (CTC) typically constitute one cancer cell per billion normal blood cells and present an important tool for cancer diagnosis2,3,4. However, CTC detection strategies rely on antibody-mediated capture targeting cell-surface expression of the epithelial cell adhesion molecule (EpCAM), which is not present on GBM cells4. Previous studies have demonstrated the use of a microfluidic device that can be used to deplete leukocytes and enrich CTC in an antigen-agnostic manner. In this study, we used a unique technology of immunomagnetic separation paired with fluorescent labeling of CD146 expression to enrich CTC in animal models of orthotopic patient-derived xenografts. We demonstrate the successful use of a CTC technology approach to the detection of cell surface target molecules that has the potential to be translated into clinical applications to select patients appropriate for the targeted therapy.
Engineered stem cell therapeutics present a promising therapeutic strategy for GBM5,6,7,8. Although much has been studied on the utilization of autologous cell-based approaches in various cancer types9,10,11,12, allogeneic cell-based therapies such as mesenchymal stem cells (MSC) and neural stem cells (NSC) have emerged as promising options. Considering the critical and very limited timeline from diagnosis to primary surgical intervention in GBM patients13, allogeneic “off-the-shelf” engineered stem cells offer a promising therapeutic strategy to target residual GBM post-surgery. Adult bone marrow MSC, owing to their low expression of constitutive major histocompatibility complex (MHC) 1, absence of MHC2, and co-stimulatory molecules such as CD80, CD86, and CD40, confer them to be weakly immunogenic14. This characteristic allows for the use of allogeneic MSC in patients without the need for immunosuppressive therapy14. Several studies including ours have previously shown that MSC, in response to chemotactic signals released by GBM, home to tumors and incorporate into the tumor microenvironment15,16,17. This phenomenon has been leveraged for therapeutic targeting by engineering MSC to release tumor-specific cytotoxic agents that alleviate tumor burden and prolong survival in preclinical models of GBM5,6,7,8.
Although allogeneic MSC-based therapies present potential promise in targeting various cancers including GBM, the route of administration determines how well this therapeutic approach can ultimately be utilized. Given the limited success of systemic administration of MSC-based therapies in selective cancer types18,19, local delivery of cell-based therapies presents a promising potential for the treatment of GBM as it circumvents the critical concerns of biodistribution and allows for therapeutic agents to reach the desired concentration at the target area20,21. We have previously reported that hyaluronic acid (HA)-based biodegradable semi-synthetic extracellular matrices (sECM) provide a therapeutically inert approach for introducing therapeutic MSC into the tumor resection site and prevent the washout5,6,7,8.
In this study, we developed a clinical-grade engineered bifunctional MSC expressing a cell surface receptor-targeted therapeutic and a built-in safety switch (MSCBif) for advanced pre-clinical efficacy, stability, and safety evaluation. We characterize and test clinical HyStem-C hydrogel encapsulated MSCBif (EnMSCBif) in murine models of invasive primary and recurrent GBM tumor resection that mimic the clinical scenario of GBM tumor debulking and subsequent treatment post-GBM resection. Upon validation, this study will pave the path to clinical testing in Phase I/II study in primary and recurrent GBM patient cohorts.
Results
Detection of target cell surface receptor on circulating tumor cells
The current GBM therapeutic standard of care for a primary diagnosis involves surgical debulking followed by radiotherapy and adjuvant chemotherapy with temozolomide (Fig. 1a). However, over 90% of these tumors recur and the treatment is limited to surgical debulking with or without chemotherapy. The Cancer Genome Atlas (TCGA) datasets of GBM patients reveal a mere 6% 5-year survival rate thereby creating an unmet need to develop targeted therapies (Fig. 1b). To mine for therapeutic targets, further analysis of the TCGA GBM patient genomic datasets was performed using the cBioPortal for Cancer Genomics22,23. Genomic data analysis suggested genetic alterations in various genes such as Epidermal growth factor receptor (EGFR), Tumor protein 53 (TP53), Phosphatase and Tensin Homolog (PTEN), and Tumor necrosis factor receptor superfamily genes 10A and 10B (TNFRSF10A or DR4 and TNFRSF10B or DR5) (Fig. 1c). This data is suggestive of developing receptor-targeted therapies for GBM as a potential treatment. Recent advances in technology now permit robust and reproducible detection of circulating tumor cells (CTC) from a blood test4. These isolated CTC can serve as important diagnostic markers and unravel target molecules allowing the selection of tailored therapeutics to treat malignancies such as GBM (Fig. 1d). These technological advancements can even allow CTC diagnostics to identify candidates for targeted gene therapies and integrate them into the standard of care (Fig. 1d). To develop efficient models that fully represent the GBM landscape, we compared the target-specific cell surface receptor expression in the patient tumor samples with that of xenografts generated from patient-derived cell lines grown in mice. Immunohistochemistry analysis for various cell surface receptors such as DR4, DR5, and EGFR showed diverse levels in the tumor blocks from three patient tissue samples (Fig. 1e) and corresponding GBM models derived from the patient-derived cell lines, GBM8, GBM18, and GBM31R (Fig. 1f). Comparative analysis revealed correlative receptor signatures in the tumor tissue blocks as compared to the mouse tumor xenografts (MX) (Fig. 1g). These findings validate the use of patient-derived GBM models to evaluate promising therapeutic strategies for GBM.
a Therapeutic roadmap representing the clinical standard of care for primary and recurrent GBM patients. b Kaplan–Meier survival plot showing 10-year survival data of 587 GBM patients from the TCGA portal. c Genomic mutation analysis of GBM patient datasets revealing the potential for the development of receptor-targeted therapies. d Illustration showing the diagnostic application of circulating tumor cells (CTC) based stratification for patients and the potential for integration into mainline treatment. e Photomicrographs of target receptors’ staining by immunocytochemistry of patient GBM tumor blocks. f Photomicrographs of target receptors’ staining by immunocytochemistry of mice bearing GBM xenografts corresponding to tumor blocks in e. g Plot showing a comparison of target receptor expression between human tissue blocks and GBM xenografts (MX) implanted in mice (n = 3 randomly chosen microscopic fields/ sample/ marker). Image analysis was done with ImageJ. Scale bars 100 μM (e, f); Fluorescence: mCherry expression of Xenografts; NB: Normal Brain. Data are shown as mean ± S.D.
To identify the CTC in blood circulation in GBM bearing mice, we utilized a commercially available CELLTRACKS kit system24 that immunomagnetically separates the CTC from the remainder of the blood cells and subsequently captures cells positive for CD146, a high molecular weight tumor-associated antigen, and fluorescently stains for CD105 and CD45 thereby enumerating the CD105+/CD45− populations within the CD146 expressing cells25,26. We engineered established and primary patient-derived GBM lines to express a dual imaging marker, firefly luciferase (Fluc)-mCherry (FmC), namely: GBM8-FmC, GBM18-FmC, and GBM31-FmC. The CELLTRACKS system was validated for consistent capture of CD146-positive GBM cells at a very high rate (>90%) (Supplementary Fig. 1a) using the modified GBM cells in spiked culture medium samples. We further assessed the CD146 expression in three primary GBM cell lines using flow cytometry and showed a robust expression of CD146 in all the GBM lines tested (Supplementary Fig. 1b). Next, we spiked healthy human blood samples with 1500 cells/mL GBM8-FmC, GBM18-FmC, and GBM31R-FmC tumor cells and evaluated them for capture by the CELLTRACKS system. We found that a major fraction of the spiked cells were captured by the system; the mean capture percentage for GBM8-FmC, GBM-18 FmC, and GBM31R-FmC cells was 81.3, 75.33, and 79.33%, respectively (Supplementary Fig. 1c). To compare the receptor expression patterns of GBM CTC, we isolated CTC from blood samples of mice bearing patient-derived GBM8-FmC, GBM18-FmC, and GBM31R-FmC tumors using the experimental plan outlined in Fig. 2a. The blood samples obtained at various time points of tumor growth were then processed, enumerated, and further evaluated for confirmation as represented in Fig. 2b. CTC were enumerated from blood obtained from mice bearing GBM8-FmC, GBM18-FmC, and GBM31R-FmC tumors collected on day 21 post tumor implantation. The capture rates of CTC from the three mouse GBM models ranged 2000–4000 CTC/mL of blood, validating the utility of the CELLTRACKS system to isolate CTC (Fig. 2c). Blood was then collected from mice bearing GBM8-FmC, GBM18-FmC, and GBM31R-FmC tumors at two different time points: day 21 and the end stage and the CTC were enumerated using a composite overlay of CD105+/CD45− and DAPI nuclear stain (Fig. 2d and Supplementary Fig. 1d).
a Illustration showing the experimental model for CTC analysis from mice bearing GBM xenografts. b Illustration showing the principle underlying CTC isolation from blood samples using CELLTRACKS system and subsequent analysis. c Plot showing CD105+/CD45− GBM CTC isolated and enumerated from mice bearing GBM tumor xenografts harvested and analyzed at day 21 (n = 3 mice/group). d Photomicrographs showing CD105 and CD45 staining on single cells as obtained from the analyzer. e Photomicrographs revealing mCherry Fluorescence and DR5 staining on GBM-FmC-CTC isolated from mice. f Plot showing surface expression of DR5 on GBM cells and corresponding GBM-CTC by flow cytometry. g Plot showing surface expression of DR4 on GBM cells and their corresponding GBM-CTC. h Schematic depicting the workflow for CTC isolation and analysis for clinical blood samples. i Plot showing the number of CD146+/CD105+/CD45− CTC isolated from various deidentified GBM patient samples prior to surgical debulking of tumor. j Plot showing cell surface DR5 receptor expression on clinical CTC samples by flow cytometry. Scale bars 50 μM (d) and 100 μM (e). Data are shown as mean ± S.E.M.
Surface expression of DR4 and/or DR5 are efficient predictive biomarkers for identifying patient cohorts that are responsive to death receptor-targeted agents. The selective domination of either DR4 or DR5 in a particular cancer type has not been thoroughly established however, previous studies suggest the prominence of DR4 in colon and breast cancers while DR5 is better correlated as a target for GBM27,28,29,30,31,32. Immunocytochemistry of these samples confirmed the presence of tumor cells by mCherry and revealed varying levels of DR5 expression in the GBM8, GBM18, and GBM31R CTC (Fig. 2e). Flow cytometry analysis of cell surface DR5 receptor expression in parental GBM-FmC and the GBM-FmC-CTC confirmed the immunocytochemistry data (Fig. 2f and Supplementary Fig. 6a). We also found comparable levels of DR4 receptor expression on parental GBM-FmC and the GBM-FmC-CTC by flow cytometry (Fig. 2g and Supplementary Fig. 6a). These data show target receptor expression on the cell surface of parental GBM and their corresponding CTC.
To further demonstrate the feasibility of incorporating this strategy in clinical settings, we assessed the expression of one of the target receptors, DR5 in CTC isolated human blood samples that were obtained under consent prior to GBM debulking surgery (Fig. 2h). CD105+/CD45− CTC were captured in varying numbers from our samples tested (Fig. 2i). A minimum capture rate was set at 100 cells/ mL for a sample to be considered positive for capture. Four out of five samples, namely, A03, A06, A08, and A09 had cells captured over the minimum threshold (Fig. 2i). These captured CTC were then used to assess the DR5 cell surface receptor expression. Flow cytometry data revealed varying levels of cell surface expression of DR5 on the CTC obtained from the three of the clinical GBM patient samples (Fig. 2j). These findings provide a basis to isolate CTC from blood samples and subsequently stain them for target receptor detection.
Engineered allogeneic “off-the-shelf” therapeutic MSC retain their migratory and immunomodulatory properties
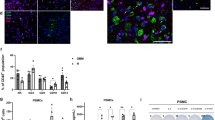
Next, we assessed the stability and therapeutic efficacy of a targeted “off-the-shelf” allogeneic-engineered MSC specifically engineered to target DR4/DR5 in GBM tumor cells (MSCBif). MSC were generated from bone marrow aspirates of qualified healthy donors under Good Manufacturing Practice (GMP) (Fig. 3a and Supplementary Fig. 2a). Specifically, eligible healthy adult donors were screened for various pathogens according to Food and Drug Administration (FDA) donor screening guidelines (21 CFR 1271.85) and were chosen for obtaining bone marrow aspirates. We engineered clinical-grade self-inactivating lentiviral (LV) vector, pRRL.PPT bearing death receptor ligand, TRAIL (DRL) under EF-1alpha promoter and the kill switch, Herpes Simplex Virus-Thymidine Kinase (HSV-TK) under the PGK promoter, along with IRES-GFP variants of these vectors to aid with pre-clinical evaluations (Supplementary Fig. 2b). GLP grade LV vectors were utilized to engineer bifunctional MSC to secrete DRL, express HSV-TK, and to further create a research cell bank of therapeutic MSC (MSCBif) to be used for characterization and subsequent evaluation. Although various engineered stem cell types have been tested for different malignancies18,33,34, the optimal stem cell type is one that offers stable secretion of the intended therapeutic agent at optimal levels. We compared the DRL release kinetics of MSCBif with human neural stem cells (NSC) and induced-pluripotent NSC (ipNSC) engineered to express DRL mediated by transduction of with LV-DRL-HSV-TK. ELISA results indicated a threefold to fivefold higher production of DRL from MSCBif as compared to the NSC and ipNSC expressing DRL (Supplementary Fig. 2c). To ensure the genetic stability of the research cell bank, we analyzed the karyotype of engineered MSC and compared it to the naïve MSC. Both MSC types demonstrated a normal human karyotype and no acquired cytogenetic changes were observed (Fig. 3b). Both naïve MSC and MSCBif expressed CD146, CD90, and CD105 and did not express CD45 validating their MSC identity (Fig. 3c, d and Supplementary Fig. 6b). Additionally, both MSC types showed a negative expression of MHC Class I chain-related proteins (MICA/B) and the MHC Class II receptor, Human Leukocyte Antigen-DR isotype (HLA-DR) (Fig. 3e, f and Supplementary Fig. 6b), confirming the immunologic inertness of MSCBif. These findings confirm that MSCBif stably retains the expression of target genes, is non-immunogenic, and provides a unique advantage in locally targeting DR4/DR5 in resected residual GBM as compared to other engineered stem cell types.
a Illustration depicting the MSC isolation and banking process. b Conventional karyotype analysis of MSCBif as compared with naive MSC showing no changes in the chromosomes post MSC transduction. c Plot showing surface expression analysis of naïve MSC and MSCBif using expression markers CD146, CD90, CD105, and CD45 revealing distinct MSC identity of MSCBif by flow cytometry. d Plot showing the quantification of various markers analyzed in c. e Immunogenicity profiling plots for MHC Class I and II antigen markers of MSC and MSCBif by flow cytometry. f Plot quantifying the immunogenicity profiling represented in e. Data shown are an aggregate of three biologically independent experiments. Data are shown as mean ± S.E.M.
sECM encapsulated MSC migrate and effectively target GSC in two-dimensional (2D) and three-dimensional (3D) models in vitro
Given that encapsulation is necessary to protect MSC from the “wash-out” by blood and cerebrospinal fluid in the tumor resection cavity5,35, we evaluated three different hydrogel systems, namely HyStem-C, Recothrom, and Vitro Gel. A modified migration assay revealed that the HA-based sECM HyStem-C encapsulated MSC migrated out of the gel more readily than the other hydrogels tested (Supplementary Fig. 3a). Next, we encapsulated MSCBif in HyStem-C hydrogel, and the resulting encapsulated MSCBif (EnMSCBif) was used for therapeutic evaluation (Fig. 4a). Time-lapse imaging of the encapsulated cells revealed efficient migration of MSCBif at the ratio of 5:5:1 of Gelin-S: Glycosil: Extralink (HyStem-C gel components) within the ranges tested in vitro (Supplementary Movie 1). A quantifiable cell migration assay was utilized to further validate the migratory potential of MSCBif in vitro. A robust migration of MSCBif was seen at a ratio of 5:5:1 of Gelin-S: Glycosil: Extralink as demonstrated by a greater population of cells traveling into the farthest zone of the cell culture well as compared to the other ratios tested (Fig. 4b, c and Supplementary Fig. 3c). We then tested the therapeutic potential of EnMSCBif on various primary patient-derived GBM stem cells (GSC) in vitro. A significant reduction in GSC viability was observed when GBM8-FmC, GBM18-FmC, and GBM31R-FmC cells were co-cultured with EnMSCBif (Fig. 4d, e) (p < 0.05 as compared to MSC-GFP) when evaluated by a bioluminescence-based assay (Fig. 4d) and a trypan blue viability assay (Fig. 4e). The functionality of the HSV-TK kill switch was evaluated using a dose-dependent treatment with the prodrug Ganciclovir (GCV) on EnMSCBif. Dose-dependent activation of the kill switch following GCV addition was seen (Supplementary Fig. 3d). Further, activation of the kill switch also contributed to an additional killing of the tumor cells owing to HSV-TK transfer mediated by cell-cell contact via the gap junctions as shown previously36 (Fig. 4d, e). Western blot analysis confirmed the cleavage of caspase-8 and resulted in poly(ADP-ribose) polymerase (PARP) cleavage in the EnMSCBif treated GSC as compared to controls, indicating potent induction of apoptotic cell death (Fig. 4f and Supplementary Fig. 7) in GBM8-FmC, GBM18-FmC, and GBM31R-FmC cell lines.
a Schematic representing the formulation of MSCBif with the hydrogel to obtain EnMSCBif. b Illustration detailing the virtual zoning strategy used for quantification of the migration assay and corresponding zones to quantitate migration. EnMSCBif was placed at the center and the migration of MSC over time was quantitated. c Plot showing migration of MSC into various zones corresponding to the illustration in b at specified time points. d Plot showing changes in viability of various GBM-FmC following EnMSCBif treatment and GCV activation of HSV-TK as compared to controls. G, GCV. Data shown for n = 3 biologically independent samples/tumor type/treatment. e Trypan blue assay revealing percent live: dead GBM cells following EnMSCBif treatment as compared to controls. G, GCV. f Western blot showing the activation of the extrinsic apoptotic cascade following EnMSCBif treatment of GBM8-FmC, GBM18-FmC, and GBM31R-FmC. TK, HSV-TK. g, h Illustration depicting the evaluation of EnMSCBif kinetics in 3D bio-printed brains (g) and bio-printed with GBM8-FmC tumors (h). i Photomicrograph of the bio-printed brain. j Photomicrographs showing changes in tumor and stem cell fluorescence over time in 3D printed brains. Statistical analyses were performed using two-way ANOVA with multiple comparisons (d, k). Data shown for n = 3 biologically independent samples/tumor type/treatment (k). Plot showing changes in GBM8-FmC survival over time following GBM resection and EnMSCBif implantation in 3D printed brains. Scale bars 100 μM (i), 50 μM (i inset) and 100 μM (j). Data are shown as mean ± S.E.M. ***p < 0.001.
To mimic the kinetics of patient GBM tumor resection in 3D settings in vitro, we bio-printed miniature brains bearing GBM8-FmC tumors (Fig. 4g, h). These bio-printed brains were followed over time by simultaneous fluorescence and bioluminescence imaging to follow changes in tumor cell growth (Fig. 4i–k). A significant reduction in the growth of GBM8-FmC in the EnMSCBif treated group was observed as compared to the control EnMSC treatment. The EnMSCBif treatment was further augmented by the addition of GCV (Fig. 4j, k). These findings validate the use of EnMSCBif as a therapeutic strategy for treating residual tumor cells in the bio-printed resection cavity and form the basis for further in vivo evaluation.
Computer-assisted modeling aids in estimating resection cavity volumes that can be mimicked in vitro
The extent of surgical debulking possible in patients with likely GBM varies depending on the tumor location in the brain. Computer-assisted 3D modeling allows for the calculation of resection cavity volumes with a potential for estimating the volume of biodegradable hydrogel required to encapsulate MSCBif for a given patient. We utilized retrospective MRI scans from 10 patients to generate pre-operative tumor volumes and subsequent resection cavity volumes using 3D Slicer program37 (Fig. 5a). The analysis revealed a major variability in cavity volumes which did not correlate with preoperative tumor volumes (Fig. 5b), suggesting that this evaluation is critical on a case-by-case basis and an intraoperative MR would provide the most accurate determination of the biodegradable hydrogel volumes for EnMSCBif formulation.
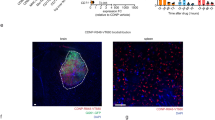
a Representative MR scan and computer modeling of tumor volume (green) and resection cavity volume (blue) using 3D slicer. b Plot representing tumor and resection cavity volumes obtained from retrospective analysis of ten deidentified GBM patients pre- and post-surgical debulking. c Illustration of the real-time formulation of GMP-MSCBif obtained from master cell bank post shipment. d Photograph of representative bags used for GMP-MSCBif shipment. e Plot showing the viability of MSCBif over time in 10%HSA. Data analyzed by comparing two groups using t test. f Plot showing gelation time of GMP-EnMSCBif with varying cell numbers. g Plot showing activation of HSV-TK kill switch in GMP-EnMSCBif following GCV administration. h Plot showing changes in tumor volume in mice bearing GBM tumors treated with varying doses of GMP-EnMSCBif post resection in a dose-escalation study. (n = 5 mice/ group). i Plot showing changes in weights of mice bearing GBM tumors treated with varying doses of GMP-EnMSCBif post resection over time. j Plot showing median gelation time of GMP-Hystem Hydrogel from the two different lots in the absence and presence of MSC. k Plot showing the viability of GBM cells following co-culture with GMP-EnMSCBif at specified time points. l Plot showing weights of mice (n = 5 mice/group) following administration of either GMP-Hystem hydrogel alone or GMP-EnMSCBif in NOD.SCID mice over time. m Photomicrographs of H&E stains of the mouse brain and other major organs following GMP-EnMSCBif administration in non-tumor-bearing mice. n Photomicrographs of brain tissues by expansion microscopy (dEXM) using GFAP and Nestin staining. Data shown for n = 3 biologically independent samples/dose (e–g). Statistical analyses were done using two-way ANOVA with multiple comparisons (h, k). Data are shown as mean ± S.E.M. Scale bars 200 μM (m), and 100 μM (n); **p < 0.05, ***p < 0.01.
Safety testing of EnMSCBif in vivo reveals no abnormalities in major organs
We then conducted IND-enabling toxicology studies similar to our intended clinical study and test runs were performed to optimize workflow. Good Manufacturing Practice (GMP) grade MSCBif were thawed and prepared from the master cell bank according to the final standard operating procedures for cell preparation and shipped in bags at 4 °C with 10% human serum albumin (HSA) from the cell manipulation facility in UC Davis, CA (Fig. 5c, d). Upon receipt of GMP-MSCBif in a temperature-controlled and stacked chamber, GMP-MSCBif were immediately transferred to a sterile biosafety cabinet and various parameters were assessed and compared to MSCBif that were cultured in house. GMP-MSCBif demonstrated over 85% viability for 72 h (Fig. 5e) and the rate of gelation of the cells when reconstituted with GMP grade (GMP)-HyStem-C was cell number dependent (Fig. 5f). Both GMP-MSCBif and MSCBif secreted similar levels of DRL over various passages (Supplementary Fig. 4a). In vitro co-culture assays of GBM cells and formulated GMP-EnMSCBif revealed that GMP-EnMSCBif like their counterparts, EnMSCBif effectively eliminated GBM8-FmC growth in vitro and the activation of the safety switch by GCV resulted in reduced MSCBif viability (Fig. 5g).
Following the validation of the shipping process and after establishing the stability of GMP-MSCBif, the second lot of GMP-MSCBif was thawed and prepared from the master cell bank according to the final standard operating procedures for cell preparation. MSCBif were shipped in bags with 10% HSA and dose escalation studies in mice bearing GBM8-FmC tumors were carried out. Specifically, NOD.SCID mice bearing GBM8- FmC tumors in cranial windows underwent fluorescence-guided tumor resection and were treated with three escalating doses of GMP-EnMSCBif and control MSC-GFP. GMP-EnMSCBif effectively reduced tumor burden in mice bearing GBM8-FmC tumors across all the three escalating doses (Fig. 5h) (p < 0.01 as compared to gel alone). Next, the safety of GMP-EnMSCBif, was assessed. No changes in weights were recorded in mice treated with GMP-EnMSCBif as opposed to a steep decline in weights of mice that received control/ sham treatments that ultimately led to humane euthanasia of these mice (Fig. 5i). No differences were seen in the rate of clinical events leading to euthanasia across all EnMSCBif groups. Histopathological analysis performed on all major organs including the brain, lungs, liver and kidneys revealed no abnormal lesions in any organs except the brain where the tumors were implanted. H&E staining of the tissue sections through each major organ except for the brain appeared normal in all mice at 2, 4, and 8 weeks (Supplementary Fig. 4b) after GMP-EnMSCBif implantation post GBM tumor resection, regardless of the dose administered. Furthermore, there were no signs of tumors in other major organs in all mice.
To evaluate any potential variability of various hydrogel lots, two different GMP-HyStem-C lots were obtained and evaluated for their physicochemical properties and stability. The two hydrogel lots tested did not reveal any differences in gelation times when constituted with GMP-MSCBif at various densities (Fig. 5j). GMP-EnMSCBif constituted with the two different lots of the hydrogel revealed a similar killing of GBM8-FmC cells in vitro (Fig. 5k). Toxicological profiling was performed to assess the safety and potential tumorigenicity of GMP-EnMSCBif in normal brain tissue. Five hundred thousand GMP-EnMSCBif were implanted into the brains of non-tumor-bearing immunodeficient mice and followed for changes in weights and any clinical signs of any adverse events. No significant variation in weights was observed in mice following GMP-EnMSCBif implantation (Supplementary Fig. 4c). Naïve mice were also evaluated for any acute toxicity or adverse events following administration of GMP-EnMSCBif constituted with the two different lots of the hydrogels. Similar to data seen earlier, the two lots did not differ in their safety profiles and showed no significant weight changes in mice (Fig. 5l). No neurological symptoms and signs (such as seizures, hemiparesis, and other movement disorders) were observed during the period following the implantation of GMP-EnMSCBif. Histopathological analysis revealed no abnormalities in the brain parenchyma or other organs (Fig. 5m). Immunofluorescence analysis of the brain tissues by expansion microscopy (dEXM) revealed normal histology of the brain by using GFAP and Nestin staining (Fig. 5n). These data reveal no significant adverse effects locally and systemically thereby validating the safety of EnMSCBif in mice.
EnMSCBif increases survival of mice in primary and recurrent mouse models of GBM resection
To assess the therapeutic efficacy of EnMSCBif in pre-clinical models of GBM resection, we implanted EnMSCBif or control sECM encapsulated MSC in the resection cavity of GBM8-FmC bearing NOD.SCID mice (Fig. 6a). Real-time intravital imaging of mice bearing GBM8-FmC tumors followed by resection and EnMSCBif implantation revealed the migration of MSC from the gel plug into the tumor site (Supplementary Fig. 5b). EnMSCBif therapy resulted in a marked decrease in tumor volumes as early as day 7 (Fig. 6b and Supplementary Fig. 5a). A further decrease in tumor burden was seen post-activation of the MSCBif kill switch via intraperitoneal injection of 10 mg/kg GCV from day 14 post EnMSCBif implantation (Fig. 6b) (p < 0.05 as compared to resection alone). This resulted in a 100% survival benefit in mice at day 150 post GBM resection (Fig. 6c) while the tumor alone group had a median survival of 49 days and resection alone, resection with MSC-GFP administration had a median survival of 91 days. To track the fate of EnMSCBif pre and post GCV treatment, we utilized clinically relevant [18F] FHBG PET imaging. A significantly high uptake of the tracer was observed in mice treated with EnMSCBif that drastically reduced following GCV treatment indicating MSCBif clearance following activation of the kill switch, HSV-TK (Fig. 6d, e). Immunofluorescence analysis on mouse brain sections revealed an increased pro-apoptotic marker, cleaved caspase 3 levels (Fig. 6f, g), in mice that received EnMSCBif as compared to control MSC-GFP.
a Schematic representing the murine model of GBM resection and treatment. b Plot showing changes in GBM8-FmC tumor volume over time following EnMSCBif administration post tumor resection as compared to controls. (n = 5 mice/group. Data representative of three independent experiments; Statistical analyses were done using two-way ANOVA with multiple comparisons). c Kaplan–Meier survival analysis of EnMSCBif treatment in GBM8-FmC mouse model of resection. d PET images showing brain uptake of [18F] FHBG tracer in mice (n = 3 mice/group) administered with EnMSCBif. e Plot representing uptake of the PET tracer, [18F] FHBG in mice administered with EnMSCBif. f Photomicrographs of immunofluorescence imaging of mouse brain sections stained with Cl. Caspase 3 as compared to control. g Plot showing the number of Cl. Caspase 3 cells as compared to control. h Plot showing changes in tumor volumes of GBM31R-FmC bearing mice (n = 5 mice/group) over time following EnMSCBif administration post tumor resection as compared to controls. i Kaplan–Meier survival analysis of EnMSCBif treatment in GBM31R-FmC mouse model of resection. Data are shown as mean ± S.E.M. Scale bars 100 μM. ***p < 0.01, ****p < 0.001.
Given that GBM recurrence is inevitable, and subsequent surgical intervention is not uncommon in clinical settings, we tested the efficacy of EnMSCBif in a patient-derived recurrent GBM model in mice. We implanted EnMSCBif or control sECM encapsulated MSC, in the resection cavity of GBM31R-FmC bearing NOD.SCID mice as described in Fig. 6a. Bioluminescence imaging revealed a significant reduction in tumor burden in mice treated with EnMSCBif as compared to untreated or control MSC treated mice (Fig. 6h) (p < 0.05 as compared to resection alone). Survival analysis revealed a 100% survival at day 90 in mice that were treated with EnMSCBif followed by activation of the HSV-TK kill switch with GCV administration (Fig. 6i) while the median survival of GBM31R-FmC bearing mice with resection alone was 55 days (Fig. 6i). These data clearly indicate the safety and therapeutic benefit of EnMSCBif combined with GCV in mice bearing highly invasive primary and recurrent GBM tumors.
Discussion
In this study, we developed a bifunctional, targeted, engineered MSC, EnMSCBif and performed advanced pre-clinical efficacy and safety profiling. This paves the path to clinical testing in Phase I/II study in GBM patient cohorts utilizing CTC-based target identification, combined with a highly selective potent biological agent to be incorporated into the clinical paradigm of GBM care at the time of surgical resection. Our results confirm a highly efficacious therapeutic profile and a favorable toxicology evaluation, thereby presenting clinical promise for a first-in-human study of EnMSCBif in GBM patients.
The incurable nature of malignant GBM can be ascribed primarily to their propensity to extensively invade brain parenchyma, making it difficult to completely resect the tumor mass38. Stratification of therapy responders is critical to the success of targeted therapeutics, particularly in GBM to improve clinical outcomes. CTC typically constitute one cancer cell per billion normal blood cells and present an important tool for cancer diagnosis4. To identify CTC in blood circulation, we chose CD146 as a marker to select these rare CTC based on a positive screen of expression in our library of primary patient-derived GBM cells. The use of CD146 as a pan-tumor cell marker has previously been validated in melanoma and is clinically approved39. Validation of CTC from GBMs involved engineering of the GBM cells to express mCherry and firefly luciferase (Fluc), spiking healthy blood samples to evaluate the validity of the cell capture and orthotopically implanting them into immune-deficient mice. We were able to capture CTC in all the three GBM models that we tested in mice, validating our approach in the preclinical models. We extended our analysis into human blood samples obtained from GBM patients and achieved CD146 selection followed by fluorescent staining to capture CD105+/CD45− CTC and detected DR5 in patient CTC samples that were tested. The low expression of cell surface DR5 seen in the limited patient CTC samples tested is in line with the data reported on varying levels of DR5 expression in many tumor types40,41,42, including GBM43,44,45.
Direct engagement of the cell surface death receptors, DR4 and DR5, with agonist or DRL directly activates the extrinsic apoptotic cascade and has provided compelling evidence for application in cancer therapy46. For GBM, about 10 therapeutic clinical trials are currently active at various phases that aim to activate the extrinsic apoptotic pathway47. Further development in this space will open avenues to automate CTC enumeration and desired stains in a continuous process to expedite the screen. Such an approach may realize the potential of a CTC-based diagnostic approach that can aid with customized treatments for a specific patient. Furthermore, the development and expansion of pan-cancer markers will open avenues for diagnostic tools for broader clinical application. To our knowledge, this is the first study that demonstrates the successful use of a CD146-based CTC capture and subsequent target receptor staining in GBM. Further research, however, is necessary to confirm that CTCs faithfully recapitulate the status of target protein expression, using matched primary tumor-CTCs in patients.
Various stem cell types such as NSCs and induced pluripotent stem cells have been previously used as therapeutic carriers for targeted tumor therapies. Immortalized NSC have previously been used for suicide gene therapeutics for brain tumors48,49,50. However, the limited availability of adult NSC and their need for immortalization limit their use as cell-based therapeutics. Although the use of an induced pluripotent stem cell platform offers the option for an autologous approach, this is labor-intensive and presents time constraints in malignancies such as GBM where there is a narrow therapeutic time window. In this study, we utilize bone marrow-derived MSC to deliver DRL that targets GBM without harming the surrounding healthy tissue. The use of MSC provides excellent stability for the validation and therapeutic application of allogeneic cell-based therapies. Our data reveal a higher and much more stable expression of DRL from MSC as compared to NSC and ipNSC. Furthermore, we show that our engineered MSC do not express HLA class I and II antigens, thus rendering them non-immunogenic. The use of allogeneic MSC provides a unique advantage of low immunogenicity paired with the ease of genetic manipulation to secrete therapeutic proteins.
Allogeneic cells can be fully characterized and banked, and therefore allow for the development of “off-the-shelf” therapeutics for various clinical conditions, including brain tumors. The utilization of such a therapeutic approach has clinical as well as commercial benefits, including product consistency, availability, and stringent quality control51,52,53,54,55,56. Studies outlined in this work were specifically performed to support an IND application, filed with the FDA to start a first-in-human (FIH) study of these engineered MSC encapsulated in a biodegradable hydrogel to assess safety and tolerability in primary and recurrent GBM patients post-surgical debulking. Specifically, we generated therapeutic MSC under GMP conditions from consented healthy donors, with the cell manufacturing occurring at the UC Davis GMP facility. This GMP facility has a decade of experience in manufacturing cell and gene therapy products for clinical trials. Expanded MSC were characterized, and genetic stability and cellular identity were confirmed. Our choice of vectors and promoters have been made in compliance with the FDA safety guidelines for lentiviral vectors. Our vectors contain a self-inactivation (SIN) deletion and include an internal promoter of choice. This approach reduces the vector’s genotoxicity and any potential phenotoxicity caused by the ectopic overexpression of transgenes potentially interfering with cell function and proliferation. Our viral vectors comprise EF-1alpha and PGK promoters that drive stable expression of transgenes57,58,59. We have utilized various in vitro systems to evaluate the therapeutic efficacy of ECM encapsulated MSCBif. The use of ECM is critical for cell retention at the tumor site and our published studies have validated the necessity of encapsulation5,35,60. To evaluate the kinetics of MSCBif in various hydrogel systems, we evaluated HyStem-C, a hyaluronic acid-based biodegradable hydrogel system, Recothrom, a human plasma-derived hydrogel, and Vitrogel, a polysaccharide hydrogel system. Based on the robust migration of MSC through the HyStem-C biodegradable hydrogel system, we utilize it for validating our therapeutic strategy. While robust migration and retention of MSC homing capabilities were primary factors for choosing an appropriate hydrogel system, immunological and therapeutic inertness and stability were other important properties. Several HA hydrogel systems have already advanced to clinical use in human and veterinary patients, as dermal fillers, for treatment of dry eye disease, as intra-articular visco-supplements, for corneal and dermal wound repair, and for post-surgical adhesion prevention, among other uses61,62. Importantly, the clinical formulation of HyStem-C, recently received Class III approval in Europe for use in adults as a resorbable matrix for delivery of autologous adipose tissue preparations to restore facial volume resulting from facial lipoatrophy (https://lineagecell.com/products-pipeline/renevia). Hystem-C has also been tested for stability following injection in the brain of higher mammals and has been reported in a 510 (k) documentation submitted by the manufacturer to the United States FDA. The ratio of various components that constitute biodegradable sECM hydrogel impacts MSC migration. Therefore, we tested various ratios of the three components constituting the hydrogel, namely, Gelin-S (a gelatin derivative), Glycosil (an HA derivative), and Extralink (the crosslinker). The most clinically favorable ratio should be the one that encapsulates the MSC and secures them within the resection bed whilst preserving their inherent migratory potential. We used a quantifiable zoning assay and time-lapse imaging to visualize the path of fluorescently labeled MSC through tumor cells in vitro which allowed for a quantifiable metric of MSC migration from the biodegradable hydrogels.
Death receptor ligands have been used to selectively target various cancers including GBM. Although DRL targets both DR4 and DR5, given the greater functional role of DR5 in GBM we evaluated the effects of the extrinsic apoptotic cascade elicited by EnMSCBif via DR5. Our results show robust activation of the extrinsic apoptotic cascade following treatment with EnMSCBif and activation of the kill switch by GCV. Our studies also revealed an additional tumor-killing effect by the activation of the HSV-TK system. This is largely due to the bystander effect of the system that has been previously reported36,63. In vivo, using animal models of brain tumor resection that closely mimic the clinical scenario of GBM management5, we tested the efficacy of EnMSCBif. In a primary patient-derived GBM xenograft model, EnMSCBif post-surgical debulking resulted in a dramatic reduction in tumor burden followed by a 100% survival in mice that received EnMSCBif and subsequent GCV. We also validated the elimination of EnMSCBif post kill switch activation using PET imaging. These data strongly support the therapeutic benefit and robust functionality of the safety switch in the MSC.
Given that safety is paramount in clinical translation, it is essential to enhance the safety of the therapeutic. Kill switches provide a unique way to safeguard therapeutics and add an additional layer of safety that can be controlled for biological therapies. Numerous kill switches have been tested and validated before; HSV-TK offers a multidimensional advantage for utilization, as it is robust at cell suicide while supplementing the therapeutic effects mediated by bystander mechanisms. Moreover, it offers a noninvasive way to track the fate of cells bearing the kill switch36,63. As an inherent safeguard for clinical use, we have incorporated the HSV-TK safety kill switch within the therapeutic vector. Our PET imaging revealed that GCV-triggered HSV-TK kill switch successfully eliminated MSCBif after their administration into the tumor resection bed. We also observed that HSV-TK conversion of prodrug GCV further augmented GBM killing. These findings are in line with the previous studies on the elimination of MSC from the brain35 and enhancing anti-tumor efficacy via bystander effects mediated by MSC-tumor cell interactions through gap junctions63.
Assessing toxicology of an agent intended for clinical use is vital for clinical translation. We have performed extensive dose-escalation studies in tumor-bearing mice to evaluate dose-limiting toxicities and single-dose toxicity studies in non-tumor-bearing mice to evaluate the effect of therapeutic components on surrounding brain tissue and any systemic adverse events. Since no established guideline is available for dose selection of cell-based therapies, we used a scaling-up calculation strategy for dose selection in mice to humans and determined that a dose of 50,000 cells in mice will correspond to 150 million cells in humans. In our clinical trial, however, we propose to use a fixed dose of 50 million cells which is less than the minimum dose we administered in mice. These calculations validate our toxicology assessments and can be extrapolated to human settings. Our data did not demonstrate any local or systemic toxicities in both tumor-bearing and non-tumor-bearing mice. Validating the toxicity profiles using various parameters is essential and further evaluation of toxicities in disease-bearing models in greater detail by independent contract research organizations (CROs) will complete an IND package for review by the FDA.
Our findings in this study build on our previous findings5 and present a comprehensive evaluation encompassing engineered MSC characterization, target detection in CTC, and preclinical efficacy of the proposed therapeutic strategy for use in GBM patients. Following evaluation of the safety and tolerability in patients with recurrent and primary GBM in a Phase I trial, subsequent clinical trials might lead to robust use of allogenic MSC therapies for hard-to-treat cancers such as GBM.
Methods
All in vivo procedures were approved by the Subcommittee on Research Animal Care at Brigham and Women’s Hospital (BWH) and Massachusetts General Hospital (MGH). Patient samples were obtained with consent under appropriate IRB approvals from Dana Farber Cancer Institute (DFCI). Healthy bone marrow aspirate samples were obtained from consented donors at the University of California at Davis Vector Production Facility.
Study design
This study was designed to evaluate the feasibility of using circulating tumor cells to screen for target receptors and utilize ligand-secreting encapsulated mesenchymal cells for GBM. This objective was addressed by: (i) determining pan-GBM markers to capture circulating tumor cells from blood and further stratify based on target receptor expression; (ii) assessing the in vitro and in vivo efficacy of the encapsulated ligand-secreting therapeutic mesenchymal stem cells; (iii) evaluating the in vivo toxicology profile of the encapsulated ligand-secreting therapeutic mesenchymal stem cells in naïve and tumor-bearing animals; and (iv) determining the clinical workflow for translating the encapsulated ligand-secreting therapeutic mesenchymal stem cells approach into humans for GBM treatment.
For in vivo studies, mice were randomized to groups according to tumor volume at the start of treatment. The number of mice per group varied between experiments and is specified in the manuscript. The primary end point was survival. To avoid investigator bias, efficacy studies in vivo were conducted in a blinded fashion where the treatments were blinded at administration and data were analyzed. All in vitro and in vivo results are representative of two to five independent experiments.
Cell lines
Patient-derived human primary invasive glioblastoma cell lines were generated and validated by Dr. Hiroaki Wakimoto at Massachusetts General Hospital. BT74, GBM4, GBM8, GBM18, and GBM31R were grown in Neurobasal medium (Life Technologies, Carlsbad, CA) supplemented with 3mM L-Glutamine (Life Technologies), B-27 supplement (Life Technologies), N-2 supplement (Life Technologies), 2 µg/ml heparin (Stem Cell Technologies, Vancouver, BC), 20 ng/ml EGF (Peprotech, Rocky Hill, NJ), and 20 ng/ml FGF (Peprotech). HEK293T cell lines were grown in DMEM supplemented with 10% (v/v) fetal bovine serum (FBS) (Life Technologies) and 1% (v/v) penicillin/streptomycin (Life Technologies). Human Mesenchymal Stem Cells were grown in DMEM supplemented with 10% (v/v) fetal bovine serum (FBS) (Life Technologies), 1% v/v L-Glutamine (Life Technologies) and 1% (v/v) penicillin/streptomycin (Life Technologies).
Viral vectors
The following therapeutic vectors: (a) LV-S-TRAIL; (b) LV-S-TRAIL-IRES-GFP; (c) LV-S- TRAIL-HSV-TK; (d) LV-S-TRAIL-HSV-TK-IRES-GFP and the diagnostic vectors LV-GFP and LV-mCherry-Fluc (FmC) were used in this study. All the therapeutic vectors were constructed in the self-inactivating lentiviral vector pRRL.PPT.EFS.GFP kindly provided by Dr. Axel Schambach (Hanover, Germany). The S- TRAIL expression is driven by the elongation factor-1α (EF1α) promoter, while the HSV-TK expression in (c) and (d) is driven by the phosphoglycerate kinase (PGK) promoter. All constructs were verified by sequencing.
Mesenchymal stem cell manufacturing
A bone marrow aspirate from a healthy adult volunteer donor was obtained following consent. Donor testing conformed to donor testing regulations as set forth in 21CFR.1271. The following tests were performed: HIV-1 and 2, HTLV 1 and 2, Hepatitis B and C, treponema pallidum, CMV, and in addition West Nile virus (due to the use of donors from Northern California). The bone marrow aspiration procedure was performed by an experienced physician specialized in hematology/oncology. The bone marrow aspirate was transported into the UC Davis GMP facility under controlled conditions. MSC were isolated by density gradient centrifugation and plastic adherence. Cells were cultured in FBS containing culture medium (Dulbecco’s Modified Eagle Medium). After appropriate MSC confluence was observed, MSC were passaged using recombinant trypsin. Passaging was kept to a minimum to retain potency. MSC were then banked and stored frozen in the vapor phase of liquid nitrogen. Appropriate testing of the banked MSC was performed, which included sterility (14-day sterility assay according to USP 71), freedom from endotoxin (limulus amebocyte lysate assay, Charles River Labs Endosafe test), and absence of mycoplasma (PCR).
Lentiviral transductions and engineering of stable cell lines
Lentiviral packaging was performed by transfection of 293T cells using second- and third-generation packaging systems. Cells were transduced with lentiviral vectors at M.O.I of 2 in a medium containing protamine sulfate (2 µg/ml). For bioluminescence imaging, cancer cells were transduced with LV-Fluc-mCherry and selected by FACS sorting or Puromycin selection (1 µg/ml) in culture. GFP or mCherry expression was visualized by fluorescence microscopy. The therapeutic transgenes were cloned into a third-generation lentiviral vector based on the pRRL.PPT.EFS.GFP backbone. The S-TRAIL expression is driven by the elongation factor-1α (EF1α) promoter, while the HSV-TK expression is driven by the phosphoglycerate kinase (PGK) promoter. The UC Davis GMP facility manufactured the lentiviral vector under Good Manufacturing Practice (GMP) conditions via transient plasmid transfection into GMP- grade HEK 293T producer cells, a human embryonic kidney cell line. The producer cell line used for vector manufacturing was tested for the absence of contaminants and human transmissible disease agents. To generate a lentiviral vector with the CCL backbone, four plasmids were manufactured and tested: the gag/pol plasmid coding for the lentiviral structural and polymerase genes, the VSV-G envelope plasmid with the gene coding for the pantropic VSV-G envelope, the transfer vector plasmid bearing S-TRAIL and HSV-TK under EF and PGK promoters, respectively, and the empty vector. These plasmids were tested for identity and purity via enzyme restriction, gel electrophoresis, and sequencing.
HEK 293T cells, initially expanded in a medium containing fetal bovine serum, were then transfected using polyethyleneimine (PEI). The growth medium was then changed to serum-free medium, and the vector was collected. Collected vector supernatant was clarified by low-speed centrifugation, DNAse-treated to remove any remaining DNA plasmids, and then concentrated via low-speed, spin-column centrifugation using columns with a defined pore size. Vector particles were retained in the columns while the liquid was expelled. The concentrated vector was then sterile filtered, aliquoted, frozen, and stored at -80 degrees Celsius until further use.
Formulation of EnMSCBif
EnMSCBif is the therapeutic product and refers to an adult allogeneic bone marrow-derived MSC product expressing secretable tumor necrosis factor apoptosis-inducing ligand (S-TRAIL) and Herpes Simplex Virus-Thymidine Kinase (HSV-TK) genes, that has been formulated with HyStem-C biodegradable hydrogel (Lineage Cell Therapeutics; Alameda, CA). The HyStem-C biodegradable hydrogel comprises of three components: Gelin-S, Glycosil, and Extralink and the final gel are constituted by mixing the three components in the ratio of 5:5:1. Specifically, MSC-TTK cells are trypsinized and pelleted, the cell pellet was suspended in required volumes of Gelin-S and Glycosil, and the required amount of Extralink was then added to form EnMSCBif, which was then ready for immediate use.
Cell viability and caspase assays
Tumor cells were plated in 96-well plates and treated with different doses of S-TRAIL for 24 h and different doses of GCV for up to 96 h. Cell viability was measured using either an ATP-dependent luminescent reagent (CellTiterGlo, Promega) for non-Fluc expressing cells or with Luciferin for Fluc-expressing cells. For the quantifiable migration assay, a concentric virtual zoning map was created within a 6-well culture plate as represented in Fig. 4b using the InCell analyzer. The migratory potential was assessed by enumerating the cell migrated into each of the zones at specified time points and represented as percent population of cells. The various zones have been color-coded in the illustration and the data for better representation. All experiments were performed in triplicate.
Co-culture experiments and time-lapse imaging
FmC engineered tumor cells (2 × 103 per well) were co-cultured with therapeutic cells in 96-well plates. For evaluation of S-TRAIL effect the relative number of Fluc-mCherry expressing tumor cells was determined by Fluc bioluminescence imaging as described 48 h later. For evaluation of the effect of GCV treatment, 10 mg/ml of Ganciclovir was added to the corresponding culture wells 48 h post MSC co-culture, and plates were read 48 h post GCV addition.
Immunoblotting
Following treatment, cells were washed with cold PBS twice, then lysed with cold RIPA buffer (20 mM Tris-HCl pH8.0, 137 mM NaCl, 10% glycerol, 1% NP-40, 0.1% SDS, 0.5% Na-deoxycholate, 2 mM EDTA pH8.0) supplemented with Complete Mini protease inhibitor (Roche Diagnostics) and phosphatase inhibitors (Phosphatase Inhibitor Cocktail II and Phosphatase Inhibitor Cocktail III from Sigma-Aldrich). Cells were scraped into 1.5 ml microtubes and centrifuged at 4 °C, 16,000 × g for 10 min. Supernatant protein concentrations were determined using a Bio-Rad protein assay kit. In all, 6× SDS-sample buffer was added to the washed samples, boiled for 3 min, and resolved by SDS-PAGE gel. For blotting whole cell lysate, 10-40 µg of protein was resolved on SDS-PAGE gel, transferred to a nitrocellulose membrane, and probed with primary antibodies.
Fluorescence-guided GBM resection and therapeutic efficacy studies
Female NOD.SCID mice, 6–8 weeks of age and 25–30 g (Charles River Laboratories, Wilmington, Massachusetts) were used for all in vivo experiments. For the establishment of clinically relevant mouse xenograft models of GBM resection, mice underwent craniotomy, tumor implantation, and surgical resection. Briefly, GBM8-FmC tumor cells (1.5 × 105 cells/mouse) or GBM31R-Fmc cells (5 × 105 cells/mouse) were implanted into the right cerebral hemisphere of mice 2 weeks after craniotomy. Bioluminescence imaging was used to follow tumor growth and mice with established tumors (day 10 post implantation) underwent fluorescence-microscopy-guided tumor resection and EnMSCBif was implanted into the resection cavity. Tumor burden was followed by Fluc imaging over time and mice were sacrificed when neurological symptoms became apparent. To evaluate therapeutic cell elimination via the inducible suicide system HSV-TK, mice bearing tumors were treated daily with an intraperitoneal injection of GCV (50 mg/kg) for 2 weeks 28 days after EnMSCBif implantation.
CTC isolation and enumeration
GBM8-FmC, GBM18-FmC, and GBM31-FmC tumor cells (1.5 × 105 cells/mouse) were implanted into the right cerebral hemisphere of NOD.SCID mice. Bioluminescence imaging was used to follow tumor growth and blood samples were obtained from the tumor-bearing mice on day 21 and at end stage by terminal cardiac puncture. For the spiked studies with cultured cells, healthy human blood was spiked with GBM8-FmC, GBM18-FmC and GBM31-FmC tumor cells at a rate of 1500 cell/mL and the samples were analyzed for the capture of CD146+ cells using the CELLTRACKS kit. Non-spiked blood samples were run in parallel to negate for any noise captured by the system and used as the background signal. CTC isolated from the blood samples were stained for death receptor 5 (DR5) by flow cytometry immediately post isolation. For human patient samples, 10 ml of blood was sampled under consent, deidentified and coded at the start of the GBM debulking surgery, and processed using the CELLTRACKS kit24 for CTC isolation and enumeration followed by flow cytometry staining for DR5 on BD Fortessa. A generous FSC/SSC gate was applied followed by a negative gate for live/dead staining for analysis. FCS files were analyzed on FlowJo (version 10.3.1).
PET imaging
Mice implanted intracranially with GBM8-FmC were fasted for 4 h prior to imaging, anesthetized with 2% isoflurane and 100% oxygen, and injected with 500–600 mCi of [18F] FHBG via the tail vein. Two hours later, a static dataset was acquired for 60 min in 1 bed position (FOV 4.2 cm) using an energy window of 250–700 keV for each mouse. Images were reconstructed using a 2D OSEM algorithm with 2 iterations and 16 subsets. For measuring the mean and maximum standardized uptake values (SUVmean and SUVmax) and metabolic tumor volume (MTV), 3D regions of interest were drawn over the tumors, and uptake values were measured using the eXplore Vista software (GE Healthcare). The maximum intensity projection images were generated using ImageJ. After the baseline PET scan at day 0, mice were treated daily with GCV (50 mg/kg) and imaged again by PET on day 10 post GCV treatment.
Tissue processing and H&E staining
Mice bearing tumors were perfused and brains were removed and sectioned for histological analysis. Brain sections on slides were washed in PBS and mounted for microscopy to be visualized for fluorescence. For H&E staining, sections were incubated with Hematoxylin and EosinY (1% alcohol), dehydrated with 95 and 100% EtOH, and mounted in xylene-based media.
Flow cytometry analysis
Cells were dissociated, washed, and re-suspended in 0.5% BSA, 2 mM EDTA solution in PBS. Cells were stained with PE-conjugated monoclonal antibodies (eBioscience) in solution at 4 °C for 30 min. Rinses were performed with 0.5% BSA, 2 mM EDTA at 4 °C. Alexa Fluor 488 or PE-conjugated isotype-specific IgG were used as control. Flow cytometry was performed using FACSAria II (BD) cell sorter and data was analyzed using the FlowJo software.
Quantification of immunohistochemistry staining
ImageJ software was utilized to carry out quantification of immunohistochemistry staining. Specifically, a threshold quantification procedure was followed wherein the various colors were deconvoluted using the parameters of the software to obtain single-colored channels. These were then quantified using the threshold parameters of the DAB staining channel and compared across groups.
Statistics and reproducibility
Data were expressed as mean ± SEM for in vivo studies and analyzed by Student’s t test when comparing two groups. Survival times of mouse groups were analyzed and compared using log-rank test. GraphPad Prism 9 software was used for all statistical analysis and to generate Kaplan–Meier survival plots. Differences were considered significant at p < 0.05 and p < 0.01. All in vitro and in vivo results are representative of two to five independent experiments.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The processed data and relevant unprocessed images for western blots are provided in the Supplementary Figures. The TCGA datasets of Glioblastoma cases (TCGA-GBM) have been utilized for analysis in this study. TCGA data are available publicly at https://portal.gdc.cancer.gov. The raw data for Figs. 2, 5, and 6 are protected and not publicly available due to data privacy policies at AMASA Therapeutics Inc. as these data reports are being prepared for submission to the FDA. Additional data requests can be made to the corresponding author. Requests for material availability of model systems but not EnMSCBif can be made to the corresponding author and AMASA Therapeutics Inc. EnMSCBif is proprietary to AMASA Therapeutics Inc. Source data are provided with this paper.
Change history
02 February 2024
Editor's note: Readers are alerted that concerns have been raised regarding the reliability of the data presented in this article. Further editorial action will be taken if appropriate once the investigation into the concerns is complete and all parties have been given an opportunity to respond in full.
References
Bianco, J. et al. On glioblastoma and the search for a cure: where do we stand? Cell. Mol. Life Sci. https://doi.org/10.1007/s00018-017-2483-3 (2017).
Chmielewska, M., Losiewicz, K., Socha, P., Mecik-Kronenberg, T. & Wasowicz, K. The application of circulating tumor cells detecting methods in veterinary oncology. Pol. J. Vet. Sci. 16, 141–151 (2013).
Hong, B. & Zu, Y. Detecting circulating tumor cells: current challenges and new trends. Theranostics 3, 377–394 (2013).
Sullivan, J. P. et al. Brain tumor cells in circulation are enriched for mesenchymal gene expression. Cancer Discov. 4, 1299–1309 (2014).
Kauer, T. M., Figueiredo, J. L., Hingtgen, S. & Shah, K. Encapsulated therapeutic stem cells implanted in the tumor resection cavity induce cell death in gliomas. Nat. Neurosci. 15, 197–204 (2011).
Power, A. T. & Bell, J. C. Cell-based delivery of oncolytic viruses: a new strategic alliance for a biological strike against cancer. Mol. Ther. 15, 660–665 (2007).
Redjal, N., Zhu, Y. & Shah, K. Combination of systemic chemotherapy with local stem cell delivered S-TRAIL in resected brain tumors. Stem Cells 33, 101–110 (2015).
Sasportas, L. S. et al. Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy. Proc. Natl Acad. Sci. USA 106, 4822–4827 (2009).
Adams, H. J. & Kwee, T. C. Prognostic value of pretransplant FDG-PET in refractory/relapsed Hodgkin lymphoma treated with autologous stem cell transplantation: systematic review and meta-analysis. Ann. Hematol. 95, 695–706 (2016).
Kondo, E. Autologous hematopoietic stem cell transplantation for diffuse large B-cell lymphoma. J. Clin. Exp. Hematop. 56, 100–108 (2016).
Martino, M. et al. Autologous stem cell transplantation in patients with multiple myeloma: an activity-based costing analysis, comparing a total inpatient model versus an early discharge model. Clin. Lymphoma Myeloma Leuk. https://doi.org/10.1016/j.clml.2017.05.018 (2017).
Zahid, U. et al. A review of autologous stem cell transplantation in lymphoma. Curr. Hematol. Malig. Rep. 12, 217–226 (2017).
Batash, R., Asna, N., Schaffer, P., Francis, N. & Schaffer, M. Glioblastoma multiforme, diagnosis and treatment; recent literature review. Curr. Med. Chem. 24, 3002–3009 (2017).
Javazon, E. H., Beggs, K. J. & Flake, A. W. Mesenchymal stem cells: paradoxes of passaging. Exp. Hematol. 32, 414–425 (2004).
Galipeau, J. & Sensebe, L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell 22, 824–833 (2018).
Mohr, A. & Zwacka, R. The future of mesenchymal stem cell-based therapeutic approaches for cancer - from cells to ghosts. Cancer Lett. 414, 239–249 (2018).
Stuckey, D. W. & Shah, K. Stem cell-based therapies for cancer treatment: separating hope from hype. Nat. Rev. Cancer 14, 683–691 (2014).
Liu, G., David, B. T., Trawczynski, M. & Fessler, R. G. Advances in pluripotent stem cells: history, mechanisms, technologies, and applications. Stem Cell Rev. Rep. 16, 3–32 (2020).
Sage, E. K., Thakrar, R. M. & Janes, S. M. Genetically modified mesenchymal stromal cells in cancer therapy. Cytotherapy 18, 1435–1445 (2016).
Jain, K. K. A critical overview of targeted therapies for glioblastoma. Front. Oncol. 8, 419 (2018).
Zhao, M., van Straten, D., Broekman, M. L. D., Preat, V. & Schiffelers, R. M. Nanocarrier-based drug combination therapy for glioblastoma. Theranostics 10, 1355–1372 (2020).
Cerami, E. et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2, 401–404 (2012).
Gao, J. et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 6, pl1 (2013).
Riethdorf, S., O’Flaherty, L., Hille, C. & Pantel, K. Clinical applications of the CellSearch platform in cancer patients. Adv. Drug Deliv. Rev. 125, 102–121 (2018).
Andree, K. C., van Dalum, G. & Terstappen, L. W. Challenges in circulating tumor cell detection by the CellSearch system. Mol. Oncol. 10, 395–407 (2016).
de Wit, S. et al. Classification of cells in CTC-enriched samples by advanced image analysis. Cancers https://doi.org/10.3390/cancers10100377 (2018).
Deng, D. & Shah, K. TRAIL of hope meeting resistance in cancer. Trends Cancer 6, 989–1001 (2020).
Kazhdan, I. & Marciniak, R. A. Death receptor 4 (DR4) efficiently kills breast cancer cells irrespective of their sensitivity to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). Cancer Gene Ther. 11, 691–698 (2004).
Setroikromo, R., Zhang, B., Reis, C. R., Mistry, R. H. & Quax, W. J. Death receptor 5 displayed on extracellular vesicles decreases TRAIL sensitivity of colon cancer cells. Front. Cell Dev. Biol. 8, 318 (2020).
Stuckey, D. W. & Shah, K. TRAIL on trial: preclinical advances in cancer therapy. Trends Mol. Med. 19, 685–694 (2013).
Twomey, J. D., Kim, S. R., Zhao, L., Bozza, W. P. & Zhang, B. Spatial dynamics of TRAIL death receptors in cancer cells. Drug Resist. Updat. 19, 13–21 (2015).
Twomey, J. D. & Zhang, B. Circulating tumor cells develop resistance to TRAIL-induced apoptosis through autophagic removal of death receptor 5: evidence from an in vitro model. Cancers https://doi.org/10.3390/cancers11010094 (2019).
Aboody, K. S. et al. Neural stem cell-mediated enzyme/prodrug therapy for glioma: preclinical studies. Sci. Transl. Med. 5, 184ra159 (2013).
Cao, P. et al. Intraperitoneal administration of neural stem cell-nanoparticle conjugates targets chemotherapy to ovarian tumors. Bioconjug. Chem. 28, 1767–1776 (2017).
Reinshagen, C. et al. CRISPR-enhanced engineering of therapy-sensitive cancer cells for self-targeting of primary and metastatic tumors. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aao3240 (2018).
Martinez-Quintanilla, J. et al. Therapeutic efficacy and fate of bimodal engineered stem cells in malignant brain tumors. Stem Cells 31, 1706–1714 (2013).
Fedorov, A. et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 30, 1323–1341 (2012).
Louis, D. N. Molecular pathology of malignant gliomas. Annu. Rev. Pathol. 1, 97–117 (2006).
Nodomi, S. et al. CD146 is a novel marker for highly tumorigenic cells and a potential therapeutic target in malignant rhabdoid tumor. Oncogene 35, 5317–5327 (2016).
Artykov, A. A. et al. Death receptors DR4 and DR5 undergo spontaneous and ligand-mediated endocytosis and recycling regardless of the sensitivity of cancer cells to TRAIL. Front. Cell Dev. Biol. 9, 733688 (2021).
Beyrath, J. et al. Synthetic ligands of death receptor 5 display a cell-selective agonistic effect at different oligomerization levels. Oncotarget 7, 64942–64956 (2016).
Wang, S. et al. First-in-human DR5 PET reveals insufficient DR5 expression in patients with gastrointestinal cancer. J. Immunother. Cancer https://doi.org/10.1136/jitc-2021-002926 (2021).
Tian, X. et al. Modulation of CCAAT/enhancer binding protein homologous protein (CHOP)-dependent DR5 expression by nelfinavir sensitizes glioblastoma multiforme cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). J. Biol. Chem. 286, 29408–29416 (2011).
Zhang, P. et al. DR5 related autophagy can promote apoptosis in gliomas after irradiation. Biochem. Biophys. Res. Commun. 522, 910–916 (2020).
Zhu, Z. C. et al. Targeting KPNB1 overcomes TRAIL resistance by regulating DR5, Mcl-1 and FLIP in glioblastoma cells. Cell Death Dis. 10, 118 (2019).
Carneiro, B. A. & El-Deiry, W. S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 17, 395–417 (2020).
Cruz Da Silva, E., Mercier, M. C., Etienne-Selloum, N., Dontenwill, M. & Choulier, L. A Systematic review of glioblastoma-targeted therapies in phases II, III, IV clinical trials. Cancers https://doi.org/10.3390/cancers13081795 (2021).
Castro-Manrreza, M. E. & Montesinos, J. J. Immunoregulation by mesenchymal stem cells: biological aspects and clinical applications. J. Immunol. Res. 2015, 394917 (2015).
Machado Cde, V., Telles, P. D. & Nascimento, I. L. Immunological characteristics of mesenchymal stem cells. Rev. Bras. Hematol. Hemoter. 35, 62–67 (2013).
Ryan, J. M., Barry, F. P., Murphy, J. M. & Mahon, B. P. Mesenchymal stem cells avoid allogeneic rejection. J. Inflamm. 2, 8 (2005).
D’Souza, N. et al. MSC and tumors: homing, differentiation, and secretion influence therapeutic potential. Adv. Biochem. Eng. Biotechnol. 130, 209–266 (2013).
Kitamura, Y. et al. Anti-EGFR VHH-armed death receptor ligand-engineered allogeneic stem cells have therapeutic efficacy in diverse brain metastatic breast cancers. Sci. Adv. https://doi.org/10.1126/sciadv.abe8671 (2021).
Levy, O. et al. Shattering barriers toward clinically meaningful MSC therapies. Sci. Adv. 6, eaba6884 (2020).
Shah, K. Mesenchymal stem cells engineered for cancer therapy. Adv. Drug Deliv. Rev. 64, 739–748 (2012).
Song, N., Scholtemeijer, M. & Shah, K. Mesenchymal stem cell immunomodulation: mechanisms and therapeutic potential. Trends Pharmacol. Sci. 41, 653–664 (2020).
Yuan, Z. et al. Cryopreservation of human mesenchymal stromal cells expressing TRAIL for human anti-cancer therapy. Cytotherapy 18, 860–869 (2016).
Garcia-Perez, L. et al. Successful preclinical development of gene therapy for recombinase-activating gene-1-deficient SCID. Mol. Ther. Methods Clin. Dev. 17, 666–682 (2020).
Lofvall, H. et al. Hematopoietic stem cell-targeted neonatal gene therapy with a clinically applicable lentiviral vector corrects osteopetrosis in oc/oc mice. Hum. Gene Ther. 30, 1395–1404 (2019).
Poletti, V. et al. Preclinical development of a lentiviral vector for gene therapy of X-linked severe combined immunodeficiency. Mol. Ther. Methods Clin. Dev. 9, 257–269 (2018).
Bhere, D. et al. Stem cells engineered during different stages of reprogramming reveal varying therapeutic efficacies. Stem Cells 36, 932–942 (2018).
Burdick, J. A. & Prestwich, G. D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 23, H41–H56 (2011).
Highley, C. B., Prestwich, G. D. & Burdick, J. A. Recent advances in hyaluronic acid hydrogels for biomedical applications. Curr. Opin. Biotechnol. 40, 35–40 (2016).
Mesnil, M. & Yamasaki, H. Bystander effect in herpes simplex virus-thymidine kinase/ganciclovir cancer gene therapy: role of gap-junctional intercellular communication. Cancer Res. 60, 3989–3999 (2000).
Acknowledgements
We would like to thank Alex Schambach for kindly providing the pRRL.ppt.EFS.GFP vector and was obtained under a Material Transfer Agreement. We would like to thank Oskar Nowicki for assistance with the Time Lapse Imaging. All illustrations have been created for this manuscript using BioRender with a license to use the illustrations (D.B., K.S.). We thank the NeuroTechnology Studio at Brigham and Women’s Hospital for providing the InCell analyzer and Mesoscope instrument access and consultation on data acquisition and data analysis. This study was supported by Research Fund (to K.S.) and AMASA Therapeutics.
Author information
Authors and Affiliations
Contributions
D.B: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing, and final approval of manuscript; S.H.C: vector design, construction and validation, collection and assembly of data; and final approval of manuscript; P.v.d.D.: collection and assembly of data; and final approval of manuscript; D.H.: collection and assembly of data and final approval of manuscript; K.G.: collection and assembly of data and final approval of manuscript; A.K.: collection and assembly of data and final approval of manuscript; J.K.: collection and assembly of data; data analysis and interpretation and final approval of manuscript; E.R.L.: collection and assembly of data; data analysis and interpretation and final approval of manuscript; C.R.: collection and assembly of data; and final approval of manuscript; V.L.: collection and assembly of data; and final approval of manuscript; N.N.: collection and assembly of data; and final approval of manuscript; W.L.B.: provision of study material, collection and assembly of data; and final approval of manuscript; F.C.: provision of study material, collection and assembly of data; and final approval of manuscript; H.L.: provision of study material, collection and assembly of data; and final approval of manuscript; Y.S.Z.: provision of study material, collection and assembly of data; and final approval of manuscript; S.H.L.: collection and assembly of data; and final approval of manuscript; N.V.: collection and assembly of data; and final approval of manuscript; W.I.E.: provision of study material, collection and assembly of data; and final approval of manuscript; P.V.Q.: provision of study material, collection and assembly of data; and final approval of manuscript; A.G.: provision of study material, collection and assembly of data; and final approval of manuscript; N.B.: collection and assembly of data; and final approval of manuscript; A.P.: collection and assembly of data; and final approval of manuscript; R.A.: collection and assembly of data; and final approval of manuscript; B.F.: provision of study materials; and final approval of manuscript; S.S.: provision of study materials; and final approval of manuscript; A.L.: collection and assembly of data; data analysis and interpretation; and final approval of manuscript; B.R.: manuscript editing, final approval of manuscript; A.H.: manuscript editing, final approval of manuscript; E.A.C.: provision of study material, final approval of manuscript; G.P.: manuscript editing, final approval of manuscript; H.W.: conception and design, data analysis and interpretation, and final approval of manuscript; G.B.: provision of study material, data analysis and interpretation, manuscript writing, and final approval of manuscript; K.S.: conception and design, provision of study material, data analysis and interpretation, manuscript writing, and final approval of manuscript.
Corresponding author
Ethics declarations
Competing interests
K.S. owns equity in and is a member of the Board of Directors of AMASA Therapeutics, a company developing stem cell-based therapies for cancer. D.B. owns equity in and is a consultant at AMASA Therapeutics. K.S.’s and D.B.’s interests were reviewed by Brigham and Women’s Hospital and Mass General Brigham in accordance with their conflict-of-interest policies. The other authors declare that they have no competing interests.
Peer review
Peer review information
Nature Communications thanks Luigi Aloj, Alfredo Quiñones-Hinojosa, and the other anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bhere, D., Choi, S.H., van de Donk, P. et al. Target receptor identification and subsequent treatment of resected brain tumors with encapsulated and engineered allogeneic stem cells. Nat Commun 13, 2810 (2022). https://doi.org/10.1038/s41467-022-30558-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-30558-3
This article is cited by
-
Systems Medicine for Precise Targeting of Glioblastoma
Molecular Biotechnology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.